
Covectra has been providing serialization services to the pharmaceutical industry since 2008 – one of the first to provide a cloud-hosted serial number database, processing over 2 billion serial numbers.
The U.S. pharmaceutical industry is required by federal law to serialize all prescription products as of November 2018, and by 2019 most companies in the world will also require serialization to ensure integrity in the global pharmaceutical supply chain.
Recognizing the power and potential of a 2D barcode, Covectra has been a pioneer in finding other pharmaceutical industry benefits using serial numbers, including the following.
Serialization for Opioid Adherence, Abuse, and Diversion Detection
By now, you probably know that opioid adherence is the degree to which a patient correctly follows medical advice and dosage compliance.
To detect diversion, a pharmaceutical diversion detection plan is needed. The integrity of a diversion detection plan is dependent on unit level serialization.
Along with unit level serialization, unit dose blister packs are essential because they help inhibit Opioid abuse and enable Opioid adherence.
For instance, serialized unit dose packaging allows better traceability of doses dispensed. Which, in turn, gives better visibility into abusive prescribing and dispensing practices.
Unit dose blister packs also support the CDC’s recommended day’s supply of initial opioid prescriptions for new patients, which helps reduce filling errors and decrease the introduction of counterfeit opioids.
Furthermore, DSCSA (Drug Supply Chain Security Act) serialization compliance requires manufacturers, repackagers, wholesale distributors, and dispensers that determine a product in its possession or control is an illegitimate product must notify the Food and Drug Administration (FDA or Agency) and other immediate trading partners of every single dose distributed to patients.
Single-Source providers also offer better visibility into abusive prescribing and dispensing practices and enables diversion detection.
When fully implemented, pharmaceutical capsule serialization will result in transparency, traceability, and visibility, by reducing capsule counterfeiting, diversion, and theft.
Pharmaceutical Authentication
Covectra is one of the first serialization-based solutions for track-and-trace to provide a cloud-hosted serial number database for pharmaceutical authentication.
Pharmaceutical authentication is the process of verifying the legitimacy of a pill using various features and tools, either covert or overt, which provides protection of pharmaceutical products against counterfeiting and diversion.
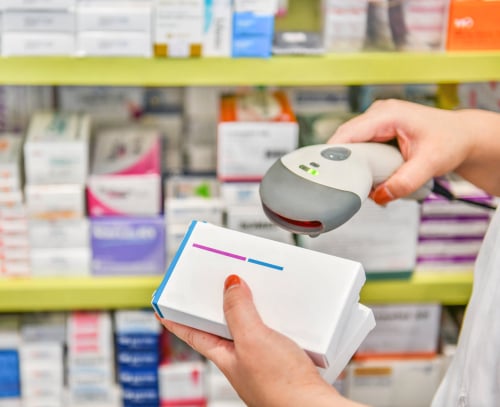
Authentication relates to the verification of a medicine’s authenticity and subsequent decommissioning using Covectra’s database cross-checking of the medicine’s unique 12-digit serial code, at the point the drug is distributed to the patient.
DSCSA legislation requires the verification of pharmaceuticals at each change of ownership without verification or decommissioning at the point of supply to the patient
Verification is key to various aspects of DSCSA compliance and is seen throughout the regulations regardless of the supply chain role as a manufacturer, repackager, wholesaler, or dispenser.
Pharmaceutical Diversion
Covectra’s track-and-trace technology is the most reliable means of combating pharmaceutical diversion because Covectra’s serialization starts at the unit dose level.
Pharmaceutical diversion is a medical and legal concept involving the transfer of any legally prescribed controlled substance from the individual for whom it was prescribed to another person for any illicit use. The term comes from the “diverting” of the drugs from their original licit medical purpose.
Diversion of pharmaceuticals can be reduced by serializing every single dose of a pharmaceutical at the unit-dose level, which Covectra offers.
To be in compliance with DSCSA track-and-trace legislation, all pharma companies must implement complete serialization throughout the pharmaceutical supply chain distribution. This will ultimately decrease diversion and help with the DSCSA identification and notification of suspect products.
Pharmaceutical Patient Communications
Pharmaceutical companies need to be exploring and leveraging the behavioral influences behind medication adherence to better target specific behaviors and audiences.
Data from Covectra’s pharmaceutical track-and-trace software proves that traceability leads to improved patient communications.
Serialization is a highly effective method for facilitating the transfer of timely and comprehensive information throughout the many functional units of a pharmaceutical enterprise including its stakeholders and, most importantly, its patients.

Additionally, to enhance overall patient experiences and outcomes, communications programs can be developed that require patients to register and offer real-time data to pharmaceutical companies using drug serial numbers.
Another key benefit of pharmaceutical product level serialization is that it enables consumer authentication, communications, and loyalty programs.
Pharmaceutical Returns Price Verification
When it comes to patients returning prescribed drugs, Covectra’s track and trace system provides return transaction authentication of pharmaceuticals, which improves chargeback payment accuracy and offers many other benefits.
Ultimately, a serialized single source provider for pharmaceuticals delivers more accurate returns management, including returns price verification.
Covectra: The Most Comprehensive Pharmaceutical Serialization Services for Your Brand
Pharmaceutical companies should obtain the maximum return on investment in addition to regulatory compliance.
Contact Covectra at info@covectra.com or give us a call at (508) 621 7320 and learn how your company can optimize that return.

